what is the final concentration of cl- when 11.5 ml of 6.0m hcl is added to 8.0 ml of water?
Chapter 3. Composition of Substances and Solutions
3.3 Molarity
Learning Objectives
By the end of this department, you will be able to:
- Describe the cardinal properties of solutions
- Summate solution concentrations using molarity
- Perform dilution calculations using the dilution equation
In preceding sections, we focused on the composition of substances: samples of thing that comprise only one type of element or compound. Notwithstanding, mixtures—samples of matter containing 2 or more substances physically combined—are more usually encountered in nature than are pure substances. Like to a pure substance, the relative composition of a mixture plays an important role in determining its properties. The relative amount of oxygen in a planet'due south atmosphere determines its ability to sustain aerobic life. The relative amounts of iron, carbon, nickel, and other elements in steel (a mixture known equally an "alloy") determine its concrete forcefulness and resistance to corrosion. The relative corporeality of the active ingredient in a medicine determines its effectiveness in achieving the desired pharmacological effect. The relative amount of sugar in a drinkable determines its sweetness (see Effigy 1). In this section, we will describe 1 of the nearly common ways in which the relative compositions of mixtures may be quantified.

Solutions
Nosotros have previously defined solutions every bit homogeneous mixtures, meaning that the limerick of the mixture (and therefore its properties) is uniform throughout its entire book. Solutions occur ofttimes in nature and accept also been implemented in many forms of manmade applied science. Nosotros will explore a more thorough handling of solution properties in the chapter on solutions and colloids, but here nosotros will introduce some of the basic properties of solutions.
The relative corporeality of a given solution component is known as its concentration. Often, though not always, a solution contains one component with a concentration that is significantly greater than that of all other components. This component is called the solvent and may be viewed equally the medium in which the other components are dispersed, or dissolved. Solutions in which water is the solvent are, of course, very mutual on our planet. A solution in which h2o is the solvent is called an aqueous solution.
A solute is a component of a solution that is typically present at a much lower concentration than the solvent. Solute concentrations are often described with qualitative terms such as dilute (of relatively low concentration) and concentrated (of relatively loftier concentration).
Concentrations may be quantitatively assessed using a wide diversity of measurement units, each convenient for particular applications. Molarity (Thousand) is a useful concentration unit for many applications in chemistry. Molarity is divers as the number of moles of solute in exactly ane liter (one L) of the solution:
[latex]M = \frac{\text{mol solute}}{\text{L solution}}[/latex]
Case i
Calculating Molar Concentrations
A 355-mL soft potable sample contains 0.133 mol of sucrose (table saccharide). What is the molar concentration of sucrose in the beverage?
Solution
Since the molar amount of solute and the book of solution are both given, the molarity tin be calculated using the definition of molarity. Per this definition, the solution book must exist converted from mL to Fifty:
[latex]M = \frac{\text{mol solute}}{\text{L solution}} = \frac{0.133 \;\text{mol}}{355 \;\text{mL} \times \frac{1 \;\text{L}}{1000 \;\text{mL}}} = 0.375 \; M[/latex]
Bank check Your Learning
A teaspoon of table carbohydrate contains about 0.01 mol sucrose. What is the molarity of sucrose if a teaspoon of sugar has been dissolved in a cup of tea with a book of 200 mL?
Example 2
Deriving Moles and Volumes from Molar Concentrations
How much sugar (mol) is contained in a small-scale sip (~10 mL) of the soft drinkable from Example 1?
Solution
In this example, we can rearrange the definition of molarity to isolate the quantity sought, moles of sugar. We then substitute the value for molarity that we derived in Example ane, 0.375 M:
[latex]Grand = \frac{\text{mol solute}}{\text{50 solution}}[/latex]
[latex]\text{mol solute} = Thousand \times \text{L solution}[/latex]
[latex]\text{mol solute} = 0.375 \;\frac{\text{mol sugar}}{\text{Fifty}} \times (ten \;\text{mL} \times \frac{1 \text{L}}{1000 \;\text{mL}}) = 0.004 \;\text{mol sugar}[/latex]
Check Your Learning
What volume (mL) of the sweetened tea described in Case 1 contains the same amount of saccharide (mol) as 10 mL of the soft drink in this case?
Instance 3
Calculating Molar Concentrations from the Mass of Solute
Distilled white vinegar (Effigy ii) is a solution of acetic acrid, CHiiiCO2H, in h2o. A 0.500-L vinegar solution contains 25.2 grand of acetic acid. What is the concentration of the acerb acid solution in units of molarity?

Solution
As in previous textbox shaded, the definition of molarity is the primary equation used to calculate the quantity sought. In this case, the mass of solute is provided instead of its molar amount, so nosotros must utilise the solute's molar mass to obtain the amount of solute in moles:
[latex]M = \frac{\text{mol solute}}{\text{L solution}} = \frac{25.2 \;\text{g CH}_3\text{CO}_2\text{H} \times \frac{1 \;\text{mol CH}_2\text{CO}_2\text{H}}{sixty.052 \;\text{g CH}_2\text{CO}_2\text{H}}}{0.500 \;\text{L solution}} = 0.839 \;Yard[/latex]
[latex]\begin{array}{r @{{}={}} l} M & \frac{\text{mol solute}}{\text{L solution}} = 0.839\;M \\[1em] M & \frac{0.839 \;\text{mol solute}}{1.00 \;\text{L solution}} \end{array}[/latex]
Check Your Learning
Summate the molarity of 6.52 chiliad of CoCltwo (128.ix yard/mol) dissolved in an aqueous solution with a total volume of 75.0 mL.
Instance 4
Determining the Mass of Solute in a Given Volume of Solution
How many grams of NaCl are contained in 0.250 L of a five.30-M solution?
Solution
The volume and molarity of the solution are specified, then the amount (mol) of solute is easily computed every bit demonstrated in Case two:
[latex]M = \;\frac{\text{mol solute}}{\text{L solution}}[/latex]
[latex]\text{mol solute} = M \times \text{L solution}[/latex]
[latex]\text{mol solute} = 5.30 \;\frac{\text{mol NaCl}}{\text{50}} \times 0.250 \;\text{L} = i.325 \;\text{mol NaCl}[/latex]
Finally, this molar amount is used to derive the mass of NaCl:
[latex]1.325 \;\text{mol NaCl} \times \frac{58.44 \;\text{grand NaCl}}{\text{mol NaCl}} = 77.4 \;\text{g NaCl}[/latex]
Check Your Learning
How many grams of CaCl2 (110.98 g/mol) are independent in 250.0 mL of a 0.200-M solution of calcium chloride?
When performing calculations stepwise, every bit in Example 4, it is of import to refrain from rounding any intermediate calculation results, which tin lead to rounding errors in the final upshot. In Example four, the molar corporeality of NaCl computed in the first pace, 1.325 mol, would be properly rounded to 1.32 mol if it were to be reported; nevertheless, although the concluding digit (5) is non pregnant, it must exist retained as a guard digit in the intermediate calculation. If we had not retained this guard digit, the final calculation for the mass of NaCl would have been 77.1 1000, a difference of 0.3 g.
In add-on to retaining a baby-sit digit for intermediate calculations, we can also avert rounding errors by performing computations in a single step (run across Example 5). This eliminates intermediate steps so that only the last result is rounded.
Example five
Determining the Volume of Solution Containing a Given Mass of Solute
In Example 3, we found the typical concentration of vinegar to be 0.839 M. What book of vinegar contains 75.vi g of acetic acid?
Solution
Outset, use the tooth mass to summate moles of acerb acrid from the given mass:
[latex]\text{g solute} \times \frac{\text{mol solute}}{\text{g solute}} = \text{mol solute}[/latex]
Then, utilize the molarity of the solution to summate the volume of solution containing this tooth corporeality of solute:
[latex]\text{mol solute} \times \frac{\text{L solution}}{\text{mol solute}} = \text{L solution}[/latex]
Combining these two steps into one yields:
[latex]\text{g solute} \times \frac{\text{mol solute}}{\text{g solute}} \times \frac{\text{L solution}}{\text{mol solute}} = \text{L solution}[/latex][latex]75.6 \;\text{g CH}_3\text{CO}_2\text{H} (\frac{\text{mol CH}_3\text{CO}_2\text{H}}{60.05 \;\text{g}}) (\frac{\text{Fifty solution}}{0.839 \;\text{mol CH}_3\text{CO}_2\text{H}}) = 1.fifty \;\text{50 solution}[/latex]
Check Your Learning
What volume of a i.l-M KBr solution contains 66.0 g KBr?
Dilution of Solutions
Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For instance, we might say that a drinking glass of iced tea becomes increasingly diluted as the ice melts. The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced tea), thereby reducing the relative concentrations of the solutes that requite the beverage its taste (Figure 3).
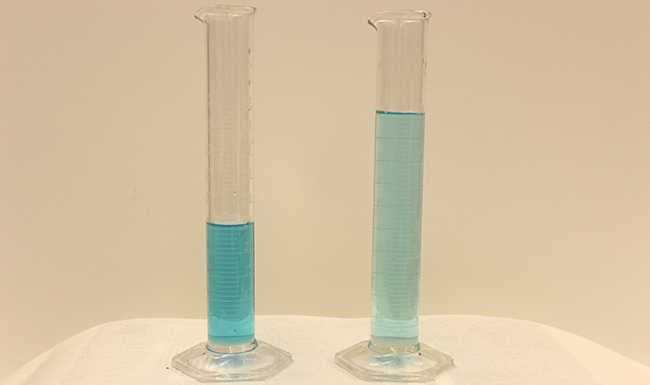
Dilution is as well a common ways of preparing solutions of a desired concentration. By adding solvent to a measured portion of a more concentrated stock solution, nosotros can achieve a item concentration. For example, commercial pesticides are typically sold equally solutions in which the agile ingredients are far more concentrated than is appropriate for their application. Before they can be used on crops, the pesticides must exist diluted. This is also a very mutual do for the training of a number of common laboratory reagents (Figure 4).

A simple mathematical relationship tin can exist used to relate the volumes and concentrations of a solution earlier and subsequently the dilution process. According to the definition of molarity, the molar corporeality of solute in a solution is equal to the product of the solution's molarity and its volume in liters:
[latex]north = ML[/latex]
Expressions like these may be written for a solution before and after it is diluted:
[latex]n_1 = M_1L_1[/latex]
[latex]n_2 = M_2L_2[/latex]
where the subscripts "1" and "2" refer to the solution before and after the dilution, respectively. Since the dilution process does non modify the amount of solute in the solution, n 1 = northward two. Thus, these ii equations may exist prepare equal to ane another:
[latex]M_1L_1 = M_2L_2[/latex]
This relation is usually referred to every bit the dilution equation. Although we derived this equation using molarity equally the unit of concentration and liters as the unit of measurement of volume, other units of concentration and book may be used, so long every bit the units properly abolish per the factor-label method. Reflecting this versatility, the dilution equation is often written in the more than full general form:
[latex]C_1V_1 = C_2V_2[/latex]
where C and Five are concentration and volume, respectively.

Use the simulation to explore the relations between solute corporeality, solution book, and concentration and to confirm the dilution equation.
Instance half-dozen
Determining the Concentration of a Diluted Solution
If 0.850 L of a 5.00-Chiliad solution of copper nitrate, Cu(NO3)2, is diluted to a volume of 1.eighty L by the addition of water, what is the molarity of the diluted solution?
Solution
We are given the volume and concentration of a stock solution, 5 one and C 1, and the book of the resultant diluted solution, V 2. We need to find the concentration of the diluted solution, C 2. We thus rearrange the dilution equation in order to isolate C two:
[latex]C_1V_1 = C_2V_2[/latex]
[latex]C_2 = \frac{C_1V_1}{V_2}[/latex]
Since the stock solution is beingness diluted by more than 2-fold (volume is increased from 0.85 L to 1.80 L), we would expect the diluted solution's concentration to be less than one-half 5 M. We will compare this ballpark estimate to the calculated result to check for any gross errors in computation (for example, such equally an improper substitution of the given quantities). Substituting the given values for the terms on the right side of this equation yields:
[latex]C_2 = \frac{0.850 \;\text{L} \times five.00 \frac{\text{mol}}{\text{L}}}{1.eighty \;\text{50}} = two.36 \;M[/latex]
This result compares well to our ballpark approximate (it's a flake less than half the stock concentration, five M).
Cheque Your Learning
What is the concentration of the solution that results from diluting 25.0 mL of a 2.04-M solution of CH3OH to 500.0 mL?
Example 7
Volume of a Diluted Solution
What book of 0.12 M HBr can be prepared from 11 mL (0.011 L) of 0.45 M HBr?
Solution
We are given the volume and concentration of a stock solution, V 1 and C 1, and the concentration of the resultant diluted solution, C 2. Nosotros need to detect the book of the diluted solution, V two. We thus rearrange the dilution equation in order to isolate V 2:
[latex]C_1V_1 = C_2V_2[/latex]
[latex]V_2 = \frac{C_1V_1}{C_2}[/latex]
Since the diluted concentration (0.12 G) is slightly more than one-fourth the original concentration (0.45 M), we would await the volume of the diluted solution to be roughly iv times the original volume, or effectually 44 mL. Substituting the given values and solving for the unknown volume yields:
[latex]V_2 = \frac{(0.45\;M)(0.011 \;\text{L})}{0.12 \; M}[/latex]
[latex]V_2 = 0.041 \;\text{L}[/latex]
The volume of the 0.12-M solution is 0.041 50 (41 mL). The issue is reasonable and compares well with our rough gauge.
Bank check Your Learning
A laboratory experiment calls for 0.125 M HNO3. What volume of 0.125 M HNO3 can be prepared from 0.250 50 of i.88 M HNO3?
Example eight
Volume of a Concentrated Solution Needed for Dilution
What volume of 1.59 M KOH is required to prepare 5.00 L of 0.100 1000 KOH?
Solution
We are given the concentration of a stock solution, C 1, and the volume and concentration of the resultant diluted solution, V 2 and C 2. We need to find the volume of the stock solution, V one. We thus rearrange the dilution equation in social club to isolate V 1:
[latex]C_1V_1 = C_2V_2[/latex]
[latex]V_2 = \frac{C_2V_2}{C_2}[/latex]
Since the concentration of the diluted solution 0.100 M is roughly one-sixteenth that of the stock solution (1.59 Chiliad), we would expect the volume of the stock solution to exist about one-sixteenth that of the diluted solution, or effectually 0.iii liters. Substituting the given values and solving for the unknown volume yields:
[latex]V_1 = \frac{(0.100\;K)(5.00 \;\text{L})}{i.59 \; M}[/latex]
[latex]V_1 = 0.314 \;\text{50}[/latex]
Thus, we would need 0.314 Fifty of the 1.59-M solution to ready the desired solution. This result is consistent with our rough gauge.
Cheque Your Learning
What book of a 0.575-M solution of glucose, C6H12O6, can be prepared from 50.00 mL of a 3.00-M glucose solution?
Key Concepts and Summary
Solutions are homogeneous mixtures. Many solutions contain ane component, called the solvent, in which other components, called solutes, are dissolved. An aqueous solution is 1 for which the solvent is water. The concentration of a solution is a measure of the relative amount of solute in a given amount of solution. Concentrations may exist measured using various units, with one very useful unit of measurement being molarity, defined as the number of moles of solute per liter of solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a elementary relation between concentrations and volumes of a solution before and after dilution.
Key Equations
- [latex]K = \frac{\text{mol solute}}{\text{L solution}}[/latex]
- C i V ane = C 2 Five ii
Chemistry End of Chapter Exercises
- Explain what changes and what stays the same when one.00 50 of a solution of NaCl is diluted to 1.lxxx L.
- What information practise we demand to calculate the molarity of a sulfuric acrid solution?
- What does it mean when we say that a 200-mL sample and a 400-mL sample of a solution of common salt take the same molarity? In what means are the two samples identical? In what ways are these two samples different?
- Determine the molarity for each of the following solutions:
(a) 0.444 mol of CoCl2 in 0.654 L of solution
(b) 98.0 thousand of phosphoric acid, H3POfour, in ane.00 L of solution
(c) 0.2074 g of calcium hydroxide, Ca(OH)two, in forty.00 mL of solution
(d) 10.5 kg of NaiiAnd thenfour·10H2O in eighteen.threescore Fifty of solution
(e) vii.0 × 10−3 mol of I2 in 100.0 mL of solution
(f) ane.8 × teniv mg of HCl in 0.075 L of solution
- Decide the molarity of each of the post-obit solutions:
(a) i.457 mol KCl in ane.500 L of solution
(b) 0.515 g of HiiAnd then4 in i.00 50 of solution
(c) 20.54 1000 of Al(NOiii)three in 1575 mL of solution
(d) 2.76 kg of CuSO4·5HtwoO in ane.45 50 of solution
(eastward) 0.005653 mol of Br2 in 10.00 mL of solution
(f) 0.000889 g of glycine, C2H5NOtwo, in i.05 mL of solution
- Consider this question: What is the mass of the solute in 0.500 L of 0.30 K glucose, CviH12Ohalf dozen, used for intravenous injection?
(a) Outline the steps necessary to answer the question.
(b) Answer the question.
- Consider this question: What is the mass of solute in 200.0 Fifty of a ane.556-M solution of KBr?
(a) Outline the steps necessary to answer the question.
(b) Reply the question.
- Summate the number of moles and the mass of the solute in each of the following solutions:
(a) 2.00 L of 18.v M H2And so4, concentrated sulfuric acrid
(b) 100.0 mL of iii.viii × 10−5 K NaCN, the minimum lethal concentration of sodium cyanide in blood serum
(c) five.50 L of 13.3 Chiliad H2CO, the formaldehyde used to "set" tissue samples
(d) 325 mL of one.8 × x−half-dozen M FeSO4, the minimum concentration of atomic number 26 sulfate detectable past sense of taste in drinking h2o
- Summate the number of moles and the mass of the solute in each of the post-obit solutions:
(a) 325 mL of viii.23 × 10−five Thousand KI, a source of iodine in the diet
(b) 75.0 mL of two.2 × 10−v Chiliad H2Soiv, a sample of acid rain
(c) 0.2500 50 of 0.1135 M Yard2CrO4, an belittling reagent used in fe assays
(d) 10.v L of 3.716 M (NH4)2SO4, a liquid fertilizer
- Consider this question: What is the molarity of KMnO4 in a solution of 0.0908 thou of KMnOiv in 0.500 50 of solution?
(a) Outline the steps necessary to answer the question.
(b) Reply the question.
- Consider this question: What is the molarity of HCl if 35.23 mL of a solution of HCl contain 0.3366 g of HCl?
(a) Outline the steps necessary to answer the question.
(b) Answer the question.
- Summate the molarity of each of the following solutions:
(a) 0.195 g of cholesterol, C27H46O, in 0.100 Fifty of serum, the average concentration of cholesterol in man serum
(b) 4.25 g of NH3 in 0.500 L of solution, the concentration of NH3 in household ammonia
(c) 1.49 kg of isopropyl alcohol, C3H7OH, in 2.50 L of solution, the concentration of isopropyl booze in rubbing booze
(d) 0.029 g of I2 in 0.100 L of solution, the solubility of I2 in h2o at 20 °C
- Calculate the molarity of each of the following solutions:
(a) 293 one thousand HCl in 666 mL of solution, a concentrated HCl solution
(b) 2.026 g FeCl3 in 0.1250 50 of a solution used every bit an unknown in general chemistry laboratories
(c) 0.001 mg Cdii+ in 0.100 L, the maximum permissible concentration of cadmium in drinking h2o
(d) 0.0079 g C7H5SNO3 in one ounce (29.6 mL), the concentration of saccharin in a diet soft drinkable.
- There is nigh ane.0 thou of calcium, every bit Ca2+, in 1.0 L of milk. What is the molarity of Caii+ in milk?
- What volume of a 1.00-M Atomic number 26(NO3)3 solution tin be diluted to set up 1.00 L of a solution with a concentration of 0.250 M?
- If 0.1718 L of a 0.3556-M C3H7OH solution is diluted to a concentration of 0.1222 Grand, what is the book of the resulting solution?
- If iv.12 L of a 0.850 M-H3PO4 solution is be diluted to a volume of 10.00 L, what is the concentration of the resulting solution?
- What volume of a 0.33-Thou C12H22O11 solution can exist diluted to prepare 25 mL of a solution with a concentration of 0.025 G?
- What is the concentration of the NaCl solution that results when 0.150 L of a 0.556-M solution is allowed to evaporate until the volume is reduced to 0.105 Fifty?
- What is the molarity of the diluted solution when each of the following solutions is diluted to the given terminal volume?
(a) 1.00 L of a 0.250-One thousand solution of Fe(NO3)3 is diluted to a final book of 2.00 L
(b) 0.5000 L of a 0.1222-M solution of C3H7OH is diluted to a final volume of 1.250 L
(c) ii.35 50 of a 0.350-K solution of H3PO4 is diluted to a last book of 4.00 L
(d) 22.50 mL of a 0.025-Thousand solution of C12H22Oxi is diluted to 100.0 mL
- What is the terminal concentration of the solution produced when 225.5 mL of a 0.09988-Chiliad solution of Na2CO3 is allowed to evaporate until the solution volume is reduced to 45.00 mL?
- A 2.00-L bottle of a solution of concentrated HCl was purchased for the general chemical science laboratory. The solution independent 868.8 g of HCl. What is the molarity of the solution?
- An experiment in a general chemical science laboratory calls for a 2.00-G solution of HCl. How many mL of eleven.9 M HCl would be required to make 250 mL of ii.00 Yard HCl?
- What volume of a 0.20-G One thousandiiSOiv solution contains 57 thou of One thousand2SO4?
- The US Environmental Protection Bureau (EPA) places limits on the quantities of toxic substances that may be discharged into the sewer organisation. Limits have been established for a variety of substances, including hexavalent chromium, which is limited to 0.50 mg/50. If an industry is discharging hexavalent chromium as potassium dichromate (K2Cr2O7), what is the maximum permissible molarity of that substance?
Glossary
- aqueous solution
- solution for which water is the solvent
- concentrated
- qualitative term for a solution containing solute at a relatively high concentration
- concentration
- quantitative measure of the relative amounts of solute and solvent present in a solution
- dilute
- qualitative term for a solution containing solute at a relatively depression concentration
- dilution
- process of adding solvent to a solution in lodge to lower the concentration of solutes
- dissolved
- describes the procedure by which solute components are dispersed in a solvent
- molarity (Chiliad)
- unit of measurement of concentration, divers as the number of moles of solute dissolved in i liter of solution
- solute
- solution component present in a concentration less than that of the solvent
- solvent
- solution component present in a concentration that is college relative to other components
Solutions
Answers to Chemistry Stop of Affiliate Exercises
2. We demand to know the number of moles of sulfuric acid dissolved in the solution and the volume of the solution.
four. (a) 0.679 K;
(b) 1.00 Thou;
(c) 0.06998 Yard;
(d) i.75 M;
(e) 0.070 Grand;
(f) half dozen.vi M
half-dozen. (a) determine the number of moles of glucose in 0.500 L of solution; determine the molar mass of glucose; make up one's mind the mass of glucose from the number of moles and its molar mass; (b) 27 g
8. (a) 37.0 mol H2SO4;
3.63 × 103 g H2SO4;
(b) 3.8 × 10−half dozen mol NaCN;
1.nine × ten−4 g NaCN;
(c) 73.2 mol H2CO;
ii.20 kg HiiCO;
(d) 5.9 × 10−7 mol FeSO4;
viii.nine × 10−5 g FeSOiv
ten. (a) Make up one's mind the molar mass of KMnOfour; determine the number of moles of KMnO4 in the solution; from the number of moles and the book of solution, make up one's mind the molarity; (b) 1.15 × 10−three M
12. (a) five.04 × 10−three Yard;
(b) 0.499 M;
(c) 9.92 M;
(d) 1.1 × x−iii M
xiv. 0.025 One thousand
sixteen. 0.5000 L
18. 1.9 mL
20. (a) 0.125 M;
(b) 0.04888 M;
(c) 0.206 M;
(e) 0.0056 1000
22. xi.9 Thousand
24. 1.half-dozen L
Source: https://opentextbc.ca/chemistry/chapter/3-3-molarity/
0 Response to "what is the final concentration of cl- when 11.5 ml of 6.0m hcl is added to 8.0 ml of water?"
إرسال تعليق